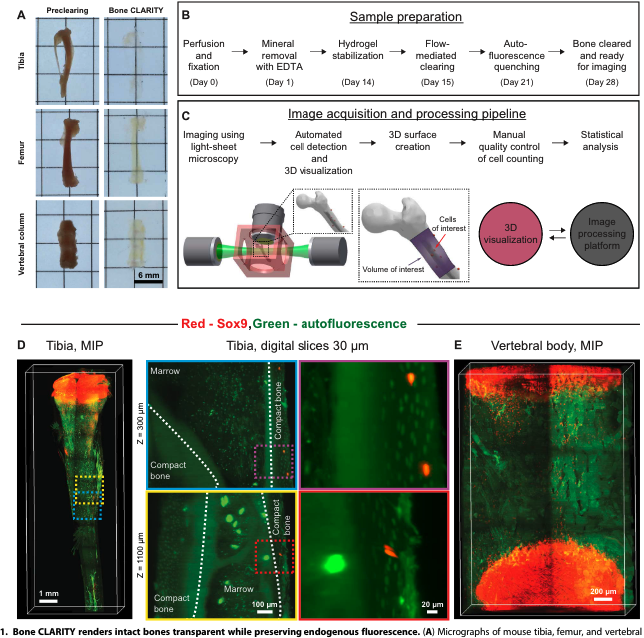
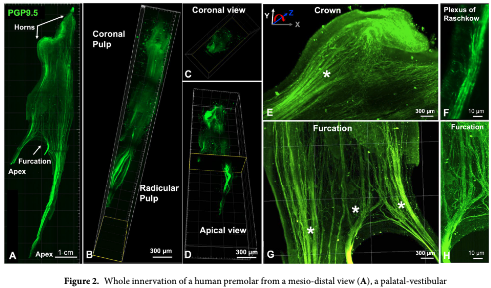
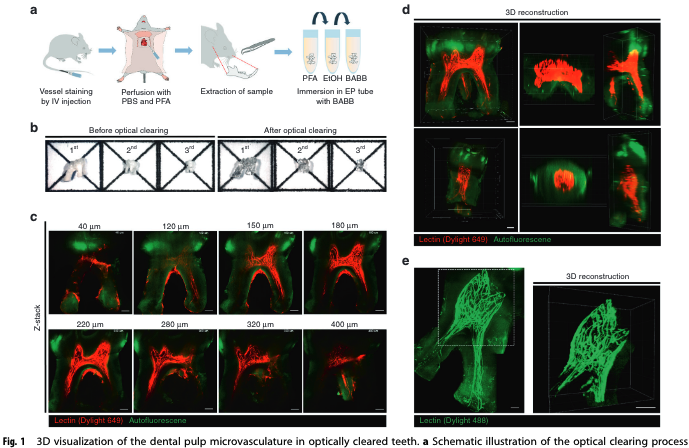
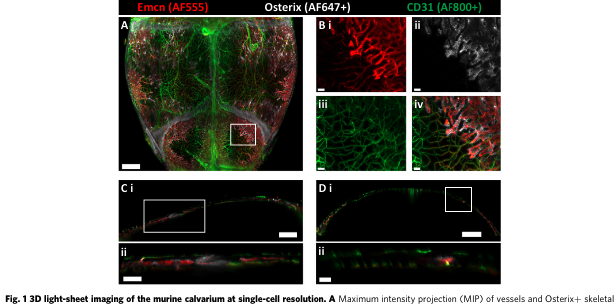
About
About descSPIM
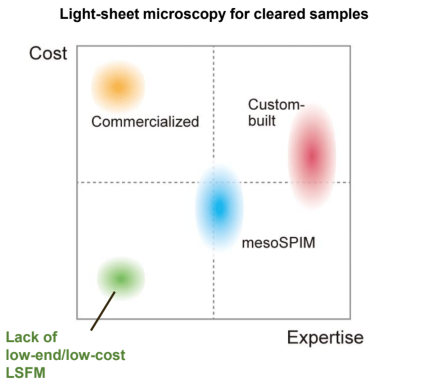
descSPIM : affordable but versatile light-sheet microscopy system for tissue clearing end users
What is descSPIM?
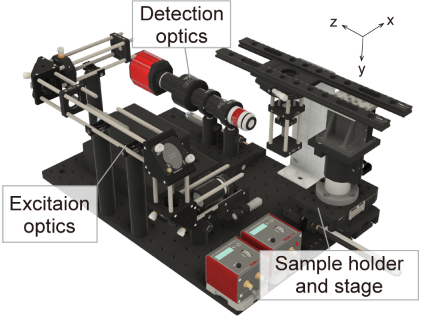
descSPIM is a lightsheet microscopy system that we designed to meet the unmet needs of researchers who are using tissue clearing techniques. The system offers a low-cost and easy-to-use solution for 3D imaging of cleared tissue samples that requires little expertise and cost. Most optical components are readily available from a single vendor and can be readily assembled using the instructions provided. These minimal optical parts are arranged on a small optical breadboard. In contrast to existing lightsheet systems, descSPIM is intended to be simple to install, build, and operate, even for end users with no prior experience in optics. descSPIM is also highly expandable and can be customized to suit a variety of applications, making it a versatile tool for a wide range of research projects. With descSPIM, researchers can easily achieve practical-quality 3D imaging of cleared specimens in a daily experiments.
The main features of descSPIM are: Easy-to-build Easy-to-operate (use a cuvette / no oil chamber) Affordable ($20k-50k for descSPIM-basic, depending on the number of lasers) Compact (all parts on a 300 mm x 450 mm breadboard)Versatile (highly expandable and customizable)## Who is the descSPIM for?
Researchers interested in using Light-sheet fluorescence microscopy (LSFM) for cleared tissue imaging
What are ideal imaging applications for a descSPIM?
- Macro- to meso-scale imaging of 3D volumetric samples prepared by tissue clearing techniques with strong efficiency (CUBIC, BABB, DISCO, SHIELDs etc.)
- Visualization of cell populations or anatomical structures (e.g. blood vessels, plaques) with cellular to sub-cellular resolutions
Guide to install
It is recommended to start learning
Basic of optics and
Add-on functions are developed to be compatible with the basic system.
Related codes can be available here: Data processing
Terms and Conditions
By using descSPIM, you agree to abide by these terms and conditions:
This system is only available for academic use. Any use for commercial purposes is strictly prohibited, unless permission is granted by the authors.
Academic users are allowed to use, apply, and adopt the system in accordance with the CC by 4.0 license policy. This includes modifications, adaptations, and redistribution of the system and its components.
If any commercial enterprise wants to use, produce, or sell the system, they must contact the authors (suishess-kyu@umin.ac.jp) to request permission.
All users of the system must acknowledge and refer to the original paper (Otomo et al. bioRxiv 2023) in any publications or presentations resulting from the use of the system.
The authors are not responsible for any consequences arising from the use of this system as well as related tools, scripts, and software. Users assume all risks and liabilities associated with the use.
The authors reserve the right to modify or terminate the system and its services at any time, without prior notice.
Users agree to comply with all applicable laws and regulations regarding the use of the system.
Any disputes arising from the use of this system shall be resolved through negotiation between the parties. If a resolution cannot be reached, the dispute shall be submitted to binding arbitration in accordance with the laws of Japan.
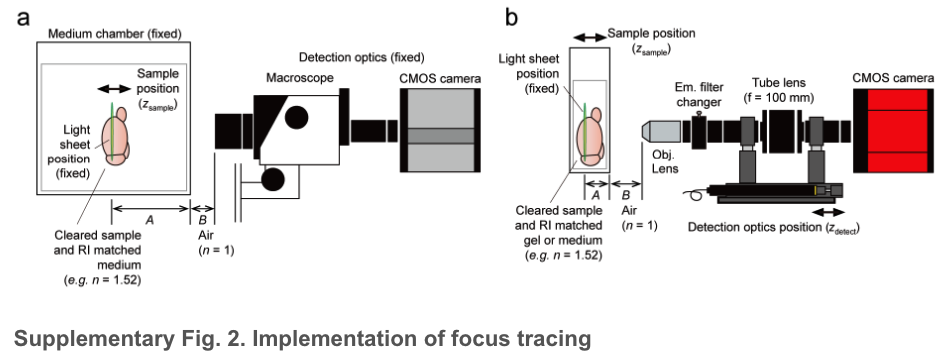
DescSPIM microscope
a. A conventional system with a positionally fixed medium chamber fulfilled with a clearing reagent or a RI-matched immersion oil (e.g., n = 1.52). The sample is moved along the z-axis within the chamber. In this case, the ratio of A (the distance from the chamber wall to the light-sheet illumination, with RI of the immersion reagent) and B (the distance from the objective lens to the chamber wall, with RI of the air (1.0)) is fixed.
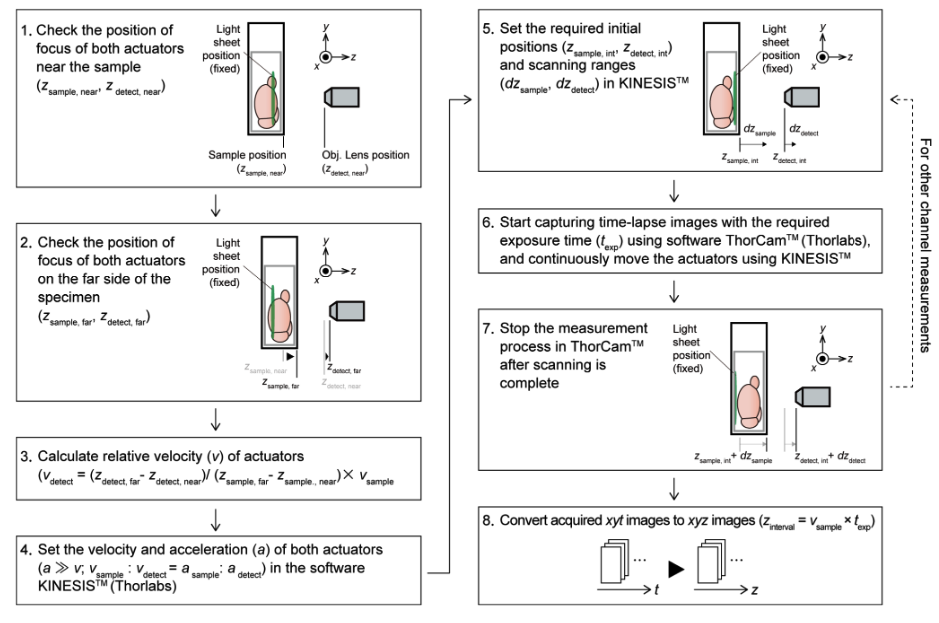
b. descSPIM sample imaging method employing a cuvette as a sample container. In this case, due to the movement of the sample chamber (the cuvette) during imaging, the ratio of A and B is altered, resulting in defocus. descSPIM applies synchronized movement of the sample stage (zsample) and the detection optics (zdetect) to prevent the defocus. See Supplementary Fig. 4 and Methods for details on how to calculate the synchronous speed correction value (the relative velocity of two actuators).
Workflow
Light Sheet Fluorescence Microscopy - Applications in research
They use BIO-33 polymer index-math to water Han et al. (2021)
short code
- Microfluidics
- Neural transports (fly wings)
- Isotropic imaging
- Functional imaging…
- Segmenting cells in a volume… (photo activate cells based on where cells are)
- Analysis of cellular adhesion, distribution and motility
- cell lineage reconstructions in complex organisms
References
---------
images and text tests:
Ex1
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aenean efficitur placerat arcu, sed feugiat ex ultrices vitae.
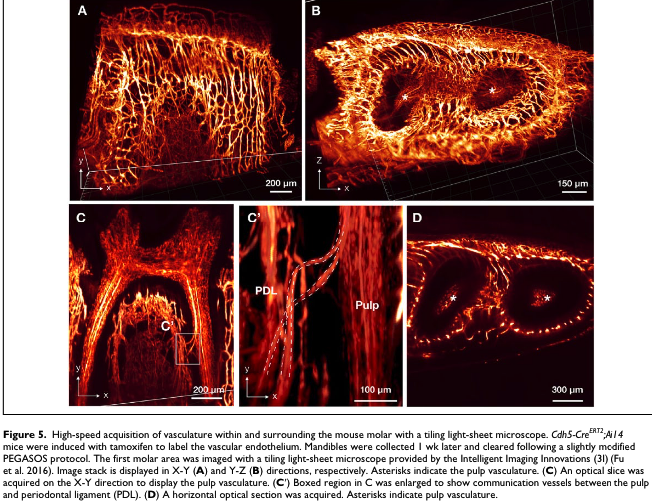
Duis sed fringilla purus. Mauris pellentesque ullamcorper justo id ullamcorper. Vestibulum finibus, mauris ac eleifend accumsan, tortor enim finibus nulla, sit amet rutrum ipsum nisl eu nunc.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vivamus et posuere mi. Sed euismod nunc ut turpis fermentum bibendum.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aenean efficitur placerat arcu, sed feugiat ex ultrices vitae.
Ex2
In rstudio visual, img floats right/left, but in render, text does not wrap around the image.
Duis sed fringilla purus. Mauris pellentesque ullamcorper justo id ullamcorper. Vestibulum finibus, mauris ac eleifend accumsan, tortor enim finibus nulla, sit amet rutrum ipsum nisl eu nunc.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Vivamus et posuere mi. Sed euismod nunc ut turpis fermentum bibendum.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Aenean efficitur placerat arcu, sed feugiat ex ultrices vitae.